Abstract
Background: Romiplostim, a TPO receptor agonist approved for adults with chronic ITP, was evaluated in children with ITP in phase 1/2 and 3 studies. Here, children with ITP are receiving open-label SC romiplostim for ≤3 years.
Methods: Eligible children, recruited in 17 countries, had ITP for ≥6 months, ≥1 prior ITP therapy, and screening platelet count ≤30×109/L or uncontrolled bleeding. Weekly SC dosing started at 1 μg/kg and was titrated weekly in 1 μg/kg increments up to 10 μg/kg to target platelet counts of 50-200×109/L. In Europe, bone marrows were evaluated at baseline and after 1 or 2 years. The primary endpoint was the % time with a platelet response in months 0-6 (response = platelet count ≥50×109/L with no rescue medications in the past 4 weeks).
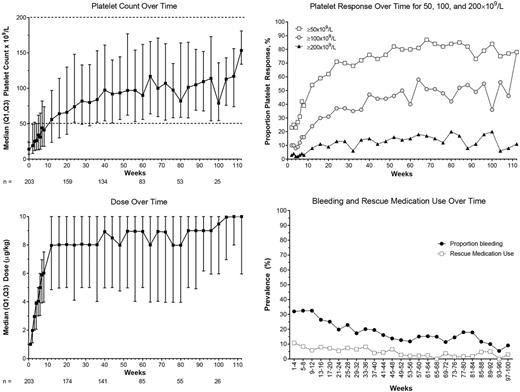
Results: As of 20 Mar 2017, 204 patients enrolled; 203 received ≥1 dose. At baseline, median (min-max) age was 10 (1-17) years; 51% were female; 10 patients (5%) had had prior splenectomy. Median (min-max) ITP duration was 1.8 (0.5-13.8) years, median baseline platelet count was 14 (2-265)×109/L, and 34 patients (17%) had baseline concurrent ITP medications. The median (Q1, Q3) % of time with a platelet response in months 0-6 was 50% (17%, 83%). Over the course of the study, 88% (179/203) of patients had a platelet response (Figure). The median (Q1, Q3) % of time with an increase in platelet counts ≥20×109/L was 74% (39%, 90%). From week 12 on, median platelet counts were >50×109/L. Platelet response and dose did not vary by age. Four patients maintained platelet counts ≥50×109/L with no ITP medications (including romiplostim) for ≥24 weeks; time to onset was 12, 14, 47, and 49 weeks after starting romiplostim. Fifty-two (26%) patients received rescue medications and 3 patients had splenectomy on study.
Median (min-max) treatment duration was 53 (8-119) weeks for a total of 226 patient-years. Median (min-max) average weekly romiplostim dose over the course of the study was 6.9 (0.2-9.5) µg/kg; the median dose was 9 μg/kg at 1 year (n = 106) and 10 μg/kg at 2 years (n = 17) (Figure). Most (63%) patients initiated self-administration. Sixty-four patients (31%) discontinued treatment; the most frequent reasons were lack of efficacy (n = 38), patient request (n = 7), adverse event (AE) (n = 7), required other therapy (n = 5), and noncompliance (n = 3). The most frequent AEs were epistaxis (32%), headache (31%), and viral upper respiratory tract infection (28%); 41 (20%) patients had serious AEs (SAEs) including epistaxis (5%), decreased platelet count (3%), and thrombocytopenia (1%). Five patients had SAEs deemed treatment-related: 2 headaches, 2 abdominal pain, and 1 each of presyncope and neutralizing antibodies (Ab). AEs leading to romiplostim discontinuation occurred in 7 patients (3%) and included 2 headaches and 1 each of abdominal pain, dizziness, interstitial lung disease, mixed connective tissue disease, neutralizing Ab, lupus, and vomiting. Bleeding was seen in 62% of patients and decreased over time. CTCAE grade ≥3 bleeding was seen in 17 patients (8%) and included epistaxis (4%), ecchymosis (1%), and contusion (1%); 2 patients had grade 4 bleeding events of "ITP".
There were 6 cases of neutralizing Ab to romiplostim (of 201 patients tested), but none to TPO; all discontinued due to Ab, 5/6 had continued elevated platelet counts, and in 2/6 cases, Ab were not found on retesting. Of 30 patients with baseline bone marrow biopsies [all with modified Bauermeister scores of grade 0 (no reticulin), 1 (fine fibers), or 2 (fine fiber network)], 27 had evaluable on-study biopsies after 1 year; 1 patient had an increase from grade 0 to 2. There was no follow-up biopsy for this patient; once at a steady dose of 10 μg/kg, most (10/16) of his platelet counts were ≥30×109/L. Four patients had an increase in 1 grade, 1 patient had a decrease in 2 grades, and 3 had a decrease in 1 grade. There were no findings of collagen or abnormalities.
Conclusion: In this interim datacut of an ongoing open-label study of romiplostim in children with ITP for ≥6 months, 88% of children had a platelet response at some point on study. Median platelet counts were ≥20×109/L above baseline ~3/4 of the time and >50×109/L from week 12 on, likely due to the time to escalate to the relatively high median dose. Overall, the median dose was 6.9 μg/kg; the median romiplostim dose over time reached 10 μg/kg. No new safety signals were observed over 226 patient-years. Future datacuts will provide more information on long-term efficacy and safety.
Grainger: GSK: Honoraria; Amgen Inc.: Honoraria; Novartis: Honoraria. Bussel: Boehringer Ingleheim: Research Funding; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; UpToDate: Honoraria, Patents & Royalties; Momenta: Membership on an entity's Board of Directors or advisory committees; Rigel: Membership on an entity's Board of Directors or advisory committees, Research Funding; Protalex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Prophylix: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Physicians Education Resource: Speakers Bureau. Tarantino: Biogen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Baxalta: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding; Grifols: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Other: Reviews grants. Cooper: National Organization for Rare Disorders: Research Funding; Imperial College BRC: Research Funding; Novartis: Consultancy, Honoraria, Speakers Bureau; GSK: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; UK ITP Support Association: Research Funding. Despotovic: Schell Cooley LLP: Other: Expert witness; Sanofi: Consultancy. Carpenter: Amgen Inc.: Employment, Equity Ownership. Eisen: Amgen Inc.: Employment, Equity Ownership. Park: Amgen Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal